
How RTU packaging accelerates time-to-market
At a time when global healthcare providers are experiencing challenges they have never had to deal with before, such as lower costs, better quality, higher flexibility, their suppliers need to react with greater speed and flexibility to meet demand without compromising patient safety. The COVID-19 pandemic is a prime example, as new products were needed immediately while maintaining costs and high quality.
This is where standardized products, such as vials, syringes and cartridges, in pre-sterilized and pre-filled packaging - referred to as ready-to-use (RTU) packaging solutions - become vital to the pharmaceutical industry. RTU packaging significantly reduces the time for drug filling and packaging, making it an ideal solution for products that must be quickly transferred from lab to clinical trials to industrialization for market use, without compromising on quality.
In this blog post, we focus on how RTU packaging helps to accelerate time-to-market, and increase cash flow and patent lifetime while maintaining superior quality and patient safety. When time-to-market (TTM) is key, three key aspects must be considered: Standardized packaging, quick changeovers, and machine compatibility.
Standardized Packaging
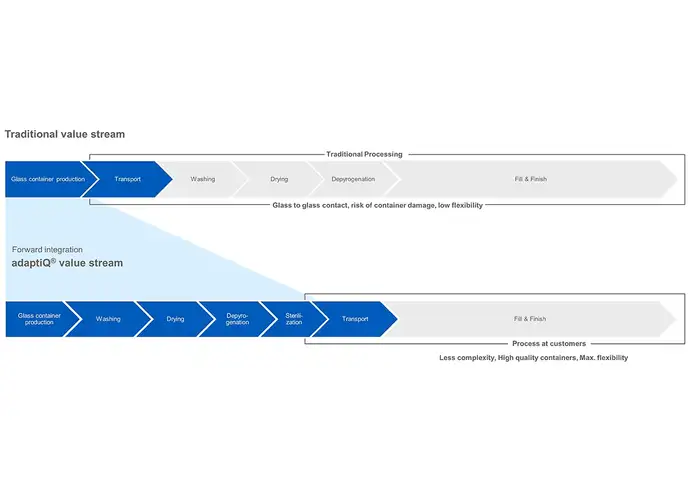
Glass containers are typically washed, dried, and depyrogenated by a pharma manufacturer before being filled and finished. This ensures that the container is safe and sterile for the fill-and-finish process. Since all container types have different dimensions, separate filling lines in separate cleanrooms are needed to handle vials, prefillable syringes and cartridges. This translates to higher investments, higher operating costs and lower flexibility.
RTU containers, however, have already undergone these processes and arrive at the manufacturer ready for filling. For a pharma company, this drastically reduces the number of steps in their operations and accelerate the time-to-market. Additionally, standardized RTU packaging allows a range of container formats to be filled on a single production line and significantly reduces cleanroom spaces and labour cost.
Quick Changeovers
Another key aspect in reducing time-to-market is the ability to speed up changeovers during processing. Packaging with different tub sizes can be a complicated and arduous process: the filling line needs to be set up manually to enable the switch to a product with different dimensions. The need to constantly change machinery is significantly reduced if standardised RTU primary packaging is available.
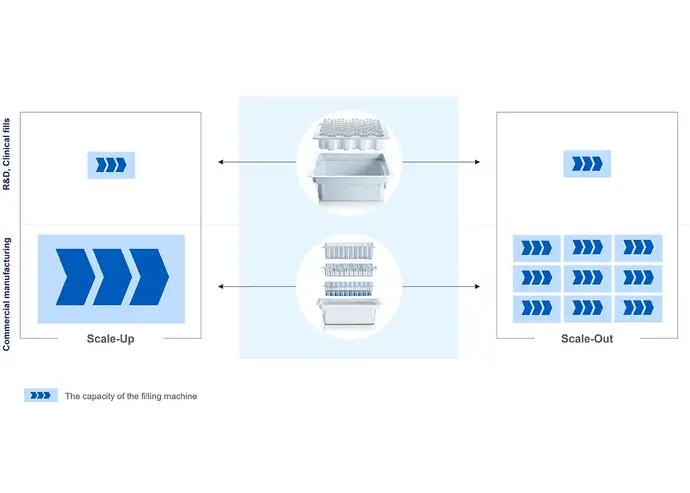
As the usage of RTU packaging results in fewer process steps that require development and validation, it can contribute to easier scale-up and scale-out. Rather than designing a customized filling line around a specific drug prior to its launch (scale-out), the pharma manufacturing company can install flexible filling capacity that can process, e.g. different vial formats on the same line (scale-up). This allows batch size variations to meet the changing market demand or manage a multi-product portfolio at a faster rate.
Machine Compatibility
The SCHOTT iQⓇ portfolio was designed to reduce complexity, enhance the flexibility of developing drug products and the filling process, and reduce time-to-market. To increase machine compatibility, SCHOTT Pharma uses standardised 3-inch tubs and standardised nests for its sterile containers, enabling simple changeovers on the filling lines and supporting scale-up and scale-out operations.
The SCHOTT iQⓇ platform is available worldwide and can be used with more than 50 machine types from a wide range of global machine manufacturers. This can also accelerate the internationalization of production by facilitating technology transfer and providing greater flexibility in expanding the production footprint. This enables easier delivery to local markets, acceleration of new filling line installation and time to market.
SCHOTT Pharma RTU Packaging
With standardised packaging, such as adaptiQⓇ as part of the SCHOTT iQⓇ platform, our customers receive vials that have already been washed and sterilised without compromising their high quality. Our main aim is to accelerate time-to-market and maximize usage of patent lifetime by entering into the market earlier with standardised packaging. Standardisation and compatibility result in quicker changeovers, reduced contamination, and the optimal time to market.
If you would like to learn more, download our whitepaper “Standardised Packaging Solutions for a fast time-to-market” or contact us.

Dr. Robert Lindner
Product Manager Bulk & Sterile Solutions
Register for the latest news
Stay up to date with information about SCHOTT Pharma products and services by registering for our newsletter.