How to choose the best ampoules for your filling line
The fill-and-finish process has continually evolved in the pharmaceutical industry, transitioning from manual operations to semi-automated and fully automated systems. A crucial component of this process is the selection of appropriate primary packaging containers, such as ampoules, vials, or syringes, each of which plays a key role in maintaining drug stability and ensuring accurate dosing.
The standardization of ampoules
Historically, the design and manufacture of ampoules have been responsive to innovations in fill-and-finish practices. The International Standard ISO 9187-1, last updated in 2010, set the standard for three primary forms of ampoules: Forms B, C, and D. This standardization ensured compatibility with evolving drug delivery systems and facilitated the uniform assessment of container closure integrity (CCI), extractables, and leachables.Unpacking the three types of ampoules
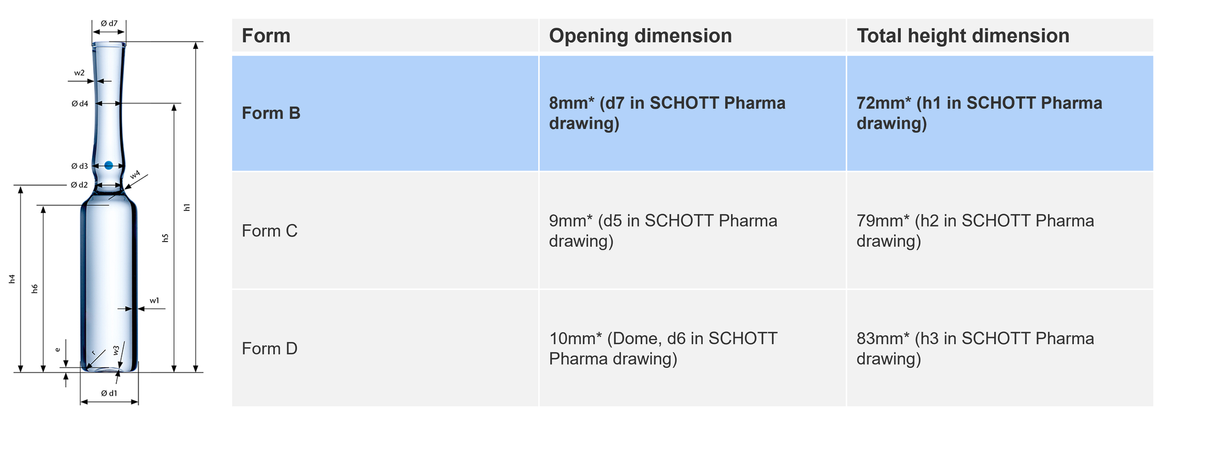
Form B ampoules: Versatility and precision
Form B ampoules, the latest in the evolution of ampoule design, were engineered with improved steam and funnel dimensions to complement modern fill-and-finish machines. They are particularly suited to fill-and-finish devices that require a precise filling needle insertion. However, form B ampoules can be used in all type of fill-and-finish machines that include a washing, drying and depyrogenation module - including old fill-and-finish machines, through a slight reduction on the line speed to achieve precision during filling.
Form B ampoules dominate the market in regions such as China, EMEA, and APAC
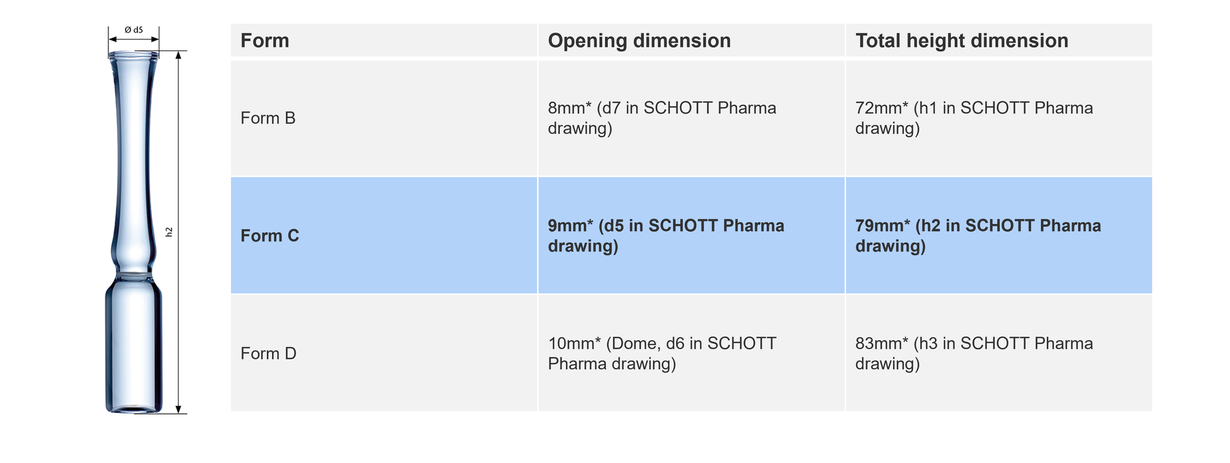
Form C ampoules: Legacy and functionality
Form C ampoules, one of the oldest types, feature a wider opening designed for compatibility with less precise filling needles. They are the preferred choice in North and Central America and parts of Asia and South America. Despite their larger dimensions than Form B, these ampoules offer a practical solution for older fill-and-finish machines. A wider opening is beneficial to avoid droplets of solution into the inner walls of the steam.
Form C ampoules distinguish themselves for having a wider opening (roughly +10%) and greater total height (roughly +10%) than form B.
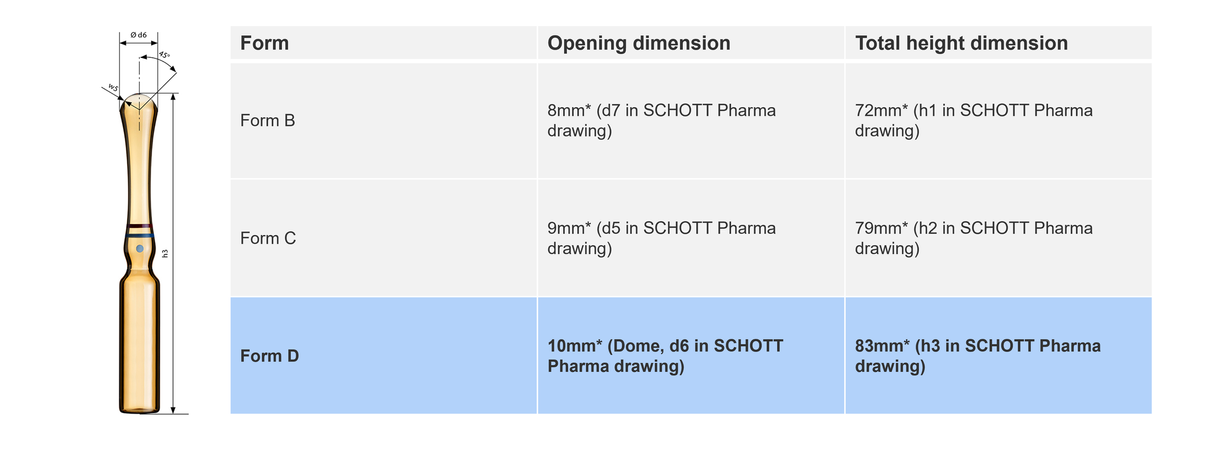
Form D ampoules, also known as closed ampoules, were designed for machines without the washing, drying, and depyrogenation module. At the time they were created, particles were not an issue, and more important was to ease the production for fill-and-finish. With the help of a burner, the CDMO or Pharmaceutical company achieves the opening of the ampoule before filling and the final closing. These ampoules continue being popular in markets such as South America and North and Central America.
The opening and total height dimensions of D ampoules are bigger than in form B and C.
Key considerations when navigating ampoule selection
Selecting the best ampoules for your fill-and-finish line involves an in-depth understanding of the different ampoule forms and their compatibility with your manufacturing process. However, choosing the right ampoule involves considerations beyond the fill-and-finish line requirements. Factors such as light sensitivity, and the risk of extractables and leachables should all be considered.SCHOTT Pharma offers you a broad portfolio which includes FIOLAX® CHR Borosilicate Glass for high sensible solutions (and/or an improved conversion process to achieve higher hydrolytic resistance levels) and different break-systems, between them, eaysOPC whose breakforce range and consistency eliminates hard to open ampoule, while protecting HCPs from injuries while opening these glass containers.
SCHOTT Pharma: Pioneering ampoule technologies
SCHOTT Pharma, a leading provider of pharmaceutical packaging solutions, offers an extensive range of ampoules designed to meet diverse fill-and-finish needs. More than 100 years of expertise in producing ampoules, allows us to adapt to all our customers’ needs, simplifying your fill-and-finish processes while decreasing your total cost of ownership.If you would like to learn more about our drug container solutions, please get in touch by filling out the contact form below.

Victoria de la Torre
Global Product Manager
Topics
Register for the latest news
Stay up to date with information about SCHOTT Pharma products and services by registering for our newsletter.