cartriQ® Large Volume Ready-to-use large-volume pharmaceutical cartridges for injectable drugs
Request quoteLarge-volume cartridges with user-friendly performance
SCHOTT Pharma’s highly accurate, sterile, and ready-to-use (RTU) cartriQ® Large Volume cartridges raise the standard of drug delivery systems. These large-volume cartridges offer reliable and patient-friendly performance, enabling the safe and simple administration of the most sensitive biologics.As part of the innovative SCHOTT iQ® platform, cartriQ® Large Volume cartridges are delivered in an industry-standard nest and tub configuration, reducing the process steps for fill-and-finish operations.
Made of highly resistant FIOLAX® clear CHR Type I Borosilicate Glass with 20% improved hydrolytic resistance and TopLine quality, these cartridges offer exceptional functionality and ease of use, offering many possibilities for modern drug delivery systems.
Exceptional drug stability and low levels of free silicone
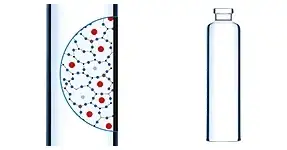
Made using SCHOTT FIOLAX® CHR Borosilicate Glass with 20% improved hydrolytic resistance, cartriQ® Large Volume cartridges offer superior chemical quality to ensure drug stability and functionality for the most sensitive biologics. A state-of-the-art siliconization process guarantees low free silicone and minimized particle load, while tight geometrical dimensions enhance dosing accuracy and performance.
High chemical stability for biologics
As part of the SCHOTT iQ® RTU platform, cartriQ® Large Volume cartridges offer a highly stable storage and self-administration solution for large-volume biologics injected over a long period of time. Dimensional customization options make them the ideal choice for advanced drug delivery systems, such as wearable on- and off-body devices and autoinjectors, combining reliability, safety, and flexibility.Available pre-sterilized and ready-to-use in a tub and nest configuration, these large-volume glass cartridges result in highly efficient fill-and-finish processes, enhancing performance and reducing contamination risks.


|

|

|
|

|
|
| Product Name |
Product Name
cartriQ® Large Volume
|
Product Name
cartriQ®
|
Product Name
Core Cartridges
|
Product Name
Cartridges Break Resistant
|
Product Name
Cartridges Double Chamber
|
| Applications |
Applications
Pre-washed and pre-sterilized for large-volume biologics
|
Applications
Pre-washed and pre-sterilized for small-volume biologics
|
Applications
Default solution for generics, chemicals and biologics
|
Applications
For emergency drugs
|
Applications
For lyophilized drugs
|
| Tubing Container Material |
Tubing Container Material
FIOLAX® CHR (controlled hydrolytic resistance)
|
Tubing Container Material
FIOLAX® CHR (controlled hydrolytic resistance)
|
Tubing Container Material
FIOLAX®
|
Tubing Container Material
FIOLAX®
|
Tubing Container Material
FIOLAX®
|
| Format |
Format
5 ml, 10 ml
|
Format
1.5 - 3 ml
|
Format
1.5 - 20 ml
|
Format
Customized upon request
|
Format
Customized upon request
|
| Quality Level |
Quality Level
TopLine
|
Quality Level
TopLine
|
Quality Level
StandardLine, TopLine
|
Quality Level
StandardLine
|
Quality Level
StandardLine
|
| Closure Systems |
Closure Systems
Stopper + Seal
|
Closure Systems
Stopper + Seal
|
Closure Systems
Stopper + Seal
|
Closure Systems
Stopper + Seal
|
Closure Systems
Stopper + Seal
|
| Pre-washed, Pre-sterilized |
Pre-washed, Pre-sterilized
Sterile available
|
Pre-washed, Pre-sterilized
Sterile available
|
Pre-washed, Pre-sterilized
Non-sterile and sterile (cartriQ®) available
|
Pre-washed, Pre-sterilized
Non-sterile available
|
Pre-washed, Pre-sterilized
Non-sterile available
|
| Packaging | Packaging | Packaging | Packaging |
Packaging
Bulk tray
|
Packaging
Bulk tray
|
| Read More | Read More | Read More | Read More |