SCHOTT Pharma and SHL Medical enhance home-based large-volume injections for patients
Wednesday, June 25, 2025, Germany, Mainz
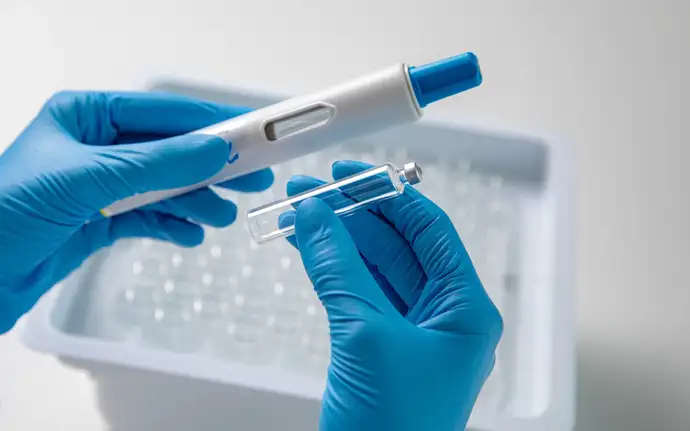
- SCHOTT Pharma and SHL Medical have joined forces to introduce the first large-volume cartridge in an autoinjector
- The development collaboration has resulted in SCHOTT Pharma’s sterile 5 ml cartriQ® cartridge, which is seamlessly compatible with SHL’s Maggie® 5.0 autoinjector
- The complete system empowers patients to self-administer substantial amounts of medication effortlessly at home, while also helping pharmaceutical companies accelerate their drugs’ market entry
Traditionally, high-viscosity drugs have been administered either intravenously in a hospital setting or, more conveniently, at home using a wearable pump system. However, because of the large doses involved and limited available delivery solutions, these administration processes can require between ten to thirty minutes. New drug delivery systems, such as large-volume handheld autoinjectors, allow medications to be comfortably self-administered at home in as little as 20 seconds, benefiting both patients and the healthcare system by reducing treatment burdens and in-clinic resources. “Recent studies show that injections with an autoinjector that last up to 60 seconds seem to be well tolerated by patients,” explains Robert Lindner, Global Product Manager Bulk and Sterile Solutions at SCHOTT Pharma.
A perfectly harmonized system
To facilitate bringing these new technologies to the market, SCHOTT Pharma has collaborated with SHL Medical to develop a large-volume RTU cartridge, compatible with a large-volume autoinjector. This innovation allows sensitive biologics in large dosages to be injected comfortably into the body in an acceptable time. Additionally, the device is user-friendly and cost-effective. “The collaboration with SCHOTT Pharma is essential to unlock the entire supply chain and expedite the market entry of large-volume combination products," says Ralph Howald, CTO at SHL Medical. "The performance flexibility of SCHOTT Pharma’s sterile 5 ml cartridge, when paired with our market-proven Needle Isolation Technology NIT®, allows us to swiftly and efficiently adjust the cannula as well as the injection times. This independence between the components is crucial in meeting diverse patient needs and enhancing the overall user experience."The 5 ml cartriQ®, combined with the new Maggie® 5.0 platform, bridges the gap between cartridge-based autoinjectors typically used for smaller volumes up to 3 ml and large-volume patch pump systems starting from 5 ml. SCHOTT Pharma’s RTU cartridge is fully compliant with ISO 21881 and features state-of-the-art baked-on silicone, mitigating the risk of contamination of sensitive drugs. The dimensions of the cartridge are designed to be fully compliant with the upcoming revision of ISO 13926-1.
More information on RTU cartridges from SCHOTT Pharma: https://www.schott-pharma.com/en/products/pharmaceutical-cartridges/cartriq-large-volume
More information on Maggie® 5.0: https://www.shl-medical.com/products/autoinjectors/maggie-50
SCHOTT Pharma’s large-volume cartridge allows sensitive biologics in large dosages to be injected comfortably into the body in an acceptable time. Image: SCHOTT Pharma/Oana Szekely
Due to their high viscosity, biologic therapies often require high drug dosages, increasing the need for large-volume solutions that are intuitive and safe for patient use. Image: SCHOTT Pharma/Oana Szekely
About SCHOTT Pharma
Human health matters. That is why SCHOTT Pharma designs solutions grounded in science to ensure that medications are safe and easy to use for people around the world. Every minute, more than 25,000 people receive an injection packed in a SCHOTT Pharma product. The portfolio comprises drug containment solutions and delivery systems for injectable drugs ranging from prefillable glass and polymer syringes to cartridges, vials, and ampoules. Every day, a team of around 4,700 people from over 60 nations works at SCHOTT Pharma to contribute to global healthcare. The company is represented in all main pharmaceutical hubs with 17 manufacturing sites in Europe, North and South America, and Asia. With over 1,000 patents and technologies developed in-house and a state-of-the-art R&D center in Switzerland, the company is focused on developing innovations for the future. SCHOTT Pharma AG & Co. KGaA is headquartered in Mainz, Germany and listed on the Frankfurt Stock Exchange as part of the SDAX. It is majority owned by SCHOTT AG, which is owned by the Carl Zeiss Foundation. In light of this spirit, SCHOTT Pharma is committed to sustainable development for society and the environment. Currently, SCHOTT Pharma has over 1,800 customers including the top 30 leading pharma manufacturers for injectable drugs and generated revenue of EUR 957 million in the fiscal year 2024. Further information at www.schott-pharma.com

Lea Kaiser
PR & Communications Manager


 Research Gate
Research Gate