Implications of transitioning mRNA Covid-19 vaccines from vial to prefilled syringe
New variations notwithstanding, the Covid-19 pandemic will inevitably evolve into an endemic situation. In many Western countries, 70-90 % of the population has now been vaccinated – an unprecedented feat that could not have happened without mass vaccination centres. The ideal container for the vaccine was the multi-dose vial, which offers a fast time-to-market backed up by extensive fill and finish experience and regulatory know-how.
From pandemic to endemic
When the disease becomes endemic, control will consist of periodic revaccinations, especially for high-risk patients. This is most likely to take place at local healthcare facilities, where the current multi-dose vial may not be the most efficient solution. This is because, once the vial is opened, the shelf life of the vaccine will be a matter of hours and any unused doses will have to be discarded.
Moving from a multi-dose to a single-dose vial or a prefilled syringe (PFS) made of polymer or glass would improve usability and reduce waste, but there are many questions that need to be answered. A PFS is a complex device with multiple components, all of which could be affected by the requirement for air transport at reduced atmospheric pressure, as well as freezing / thawing cycles and the extremely low temperature storage demanded by mRNA vaccines.
SCHOTT Pharma therefore set up an elaborate testing program using 1 ml / 1 g SCHOTT TOPPAC® syringes made from Cyclic Olefin Copolymer (COC), introducing a range of variables to gain a better understanding of the critical parameters. The syringes were filled with either water for injection (WFI) or sucrose placebo and frozen for one or two cycles at -20 °C, -50 °C and -80 °C. Three different plungers were used: standard halobutyl rubber, a partially laminated plunger, and a laminated plunger optimised for gliding forces. Filling volumes were 0.25 ml, 0.5 ml, 0.75 ml and 1 ml, and the headspace was 2 mm, 4 mm or 6 mm. The syringes were subjected to normal atmospheric pressure, as well as air transport simulation.
Plunger movement
The first question addressed is the effects on the sterile barrier, which can be breached by excessive plunger movement. Three factors have an influence on plunger movement: the amount of headspace, filling volume, and type of plunger. Different freezing temperatures and filling mediums were tested, but no difference was observed.
Plunger movement is always most critical during transportation simulation (low pressure), but choosing the right type of plunger and optimizing the fill and finish process can control this and ensure that the sterile barrier is not breached.
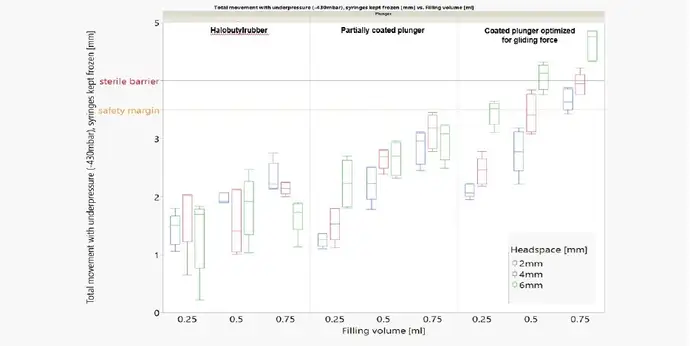
Performance comparison of syringes with a standard halobutyl rubber plunger, a partially laminated plunger, and a laminated plunger
Functionality
The next challenge is functionality, notably the effects on break loose force (BLF) and gliding forces (GF) of different temperatures over multiple freeze / thaw cycles. Over the course of three freeze / thaw cycles there was no significant difference between empty or filled syringes.
Going one step further, SCHOTT Pharma investigated an even lower temperature of -50 °C using three different plunger types: halobutyl rubber, partially coated and coated plunger optimised for gliding force. Again, there was almost no difference between -20 °C and -80 °C for both GF and BLF.
Siliconization
A further challenge is siliconization. Syringes need to have a lubrication layer inside the barrel to make plunger movement possible, but too much free silicone oil can create droplets inside the syringe that can react with the drug, causing it to be unstable.
SCHOTT Pharma tested two different siliconization technologies on SCHOTT TOPPAC® syringes: cross-linked siliconization, which is standard for SCHOTT TOPPAC®, and sprayed-on siliconization technology. Five syringes using each technology were filled with WFI and stored / frozen at different temperatures beforethe extract was pooled and analysed via Graphite Furnace Atomic Absorption for free silicone oil.
At -5 °C and -20 °C, sprayed-on silicone resulted in many times more extractable silicone than cross-linked siliconization: five time greater and 24 times greater respectively. This demonstrated that freeze / thaw cycles have a significant effect on sprayed-on silicone oil syringes.
Siliconization technology has an even bigger impact on sub-visual particles (>10 µm) at freezing temperatures, with lower temperatures seeming to increase the particle load.
Similarly, the siliconization technology also has a significant impact on particle count, with the immobilised silicone layer from cross-linked siliconization providing the lowest particle level. Silicone-free syringes in the same conditions showed no particles, so there is a high probability that all sub-visual particles observed originated from siliconization.
Container Closure integrity
Container / closure integrity may also be affected by extremely low temperatures such as -80 °C due to a loss of elasticity in the rubber components and the varying shrinkage rates of different materials. For example, it is not uncommon to see that the syringe barrel shrinks a lot less than the plunger, which could result in minimal overlap and a breach in the barrier against biologic ingress.
Testing was carried out on SCHOTT TOPPAC® 1 ml / 1 g syringes with standard halobutyl rubber components for tip cap and plunger, and compared with a range of control samples. The results showed that even at -80 °C there was no CO2 ingress after 24 hours.
Mechanical stability and optical properties were also unaffected by storage at very low temperatures, with no rise in breakage or stress cracking, and no hazing or additional visual defects.

An ideal solution
SCHOTT Pharma continues to investigate product and process variables to help mRNA manufacturers in the search for the ideal primary packaging. This includes potential drug-container interactions resulting from extractables and leachables coming from the container, and the effects of the container and storage conditions on the stability of the lipid nanoparticles used as the carrier. The company is also building a similar data package for syriQ® prefilled glass syringes.
In the meantime, this extensive data package shows that SCHOTT TOPPAC® syringes are highly suitable for mRNA vaccines.

Christoph Zauner
Head of Product Management Polymer Solutions
Register for the latest news
Stay up-to-date with information about SCHOTT Pharma products and services and register for our newsletter.