Glass delamination under full control
CONTACT USSCHOTT Pharma: Your reliable solution provider for delamination control
Borosilicate Glass has proven to be the most reliable material for storing injectable drugs. However very rare, glass delamination poses a risk for patient safety, having caused recalls of injectable drug products over the past several years.
There is no single factor that causes glass delamination in a pharmaceutical setting, and it can be a difficult problem to address because it typically doesn’t show up until the product has been stored in the container for several months. Nevertheless, there are concrete steps that pharma companies can take to minimize the risk of delamination.
Glass delamination - a rare phenomenon hard to predict
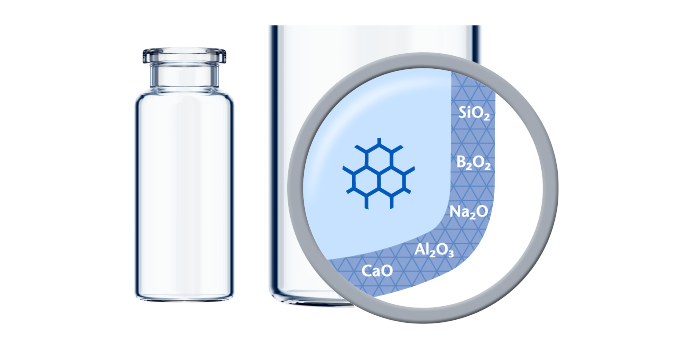
The standard glass converting process can lead to an altered surface area, the “heel zone”:
This area might be less chemically durable and therefore in general more susceptible to delamination. Delamination means the appearance of visible glass-like flakes in the drug solution after storage for a certain time period. Two different mechanisms are behind.
Delamination is not easy to predict and a phenomenon due to very rare outliers during the vial production process. Many different factors exist that influence delamination, e.g. kind of post-treatments, the drug substance and –formulation, pH, storage conditions and many more. It is key to perform predictive screening studies in advance. With their expertise in that field, SCHOTT Pharma Services can be of great help.

Glass delamination under full control with SCHOTT EVERIC® pure vials
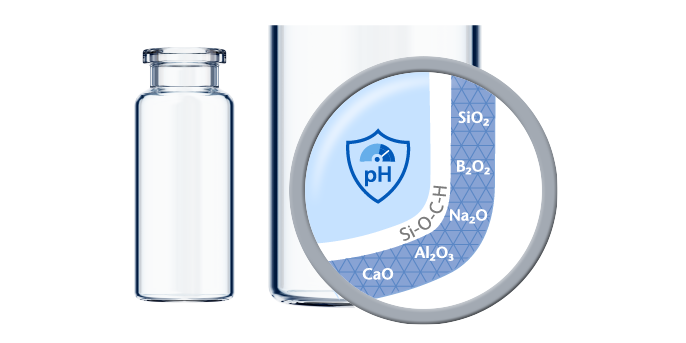
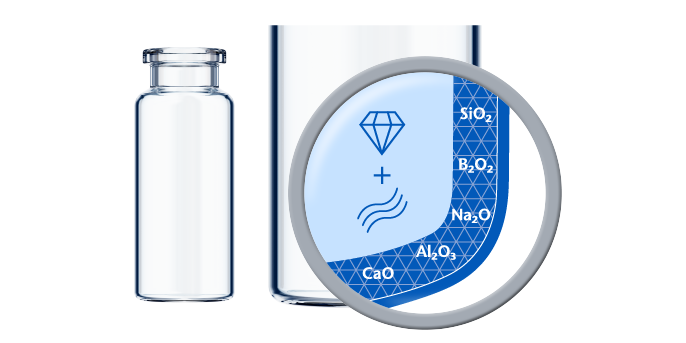
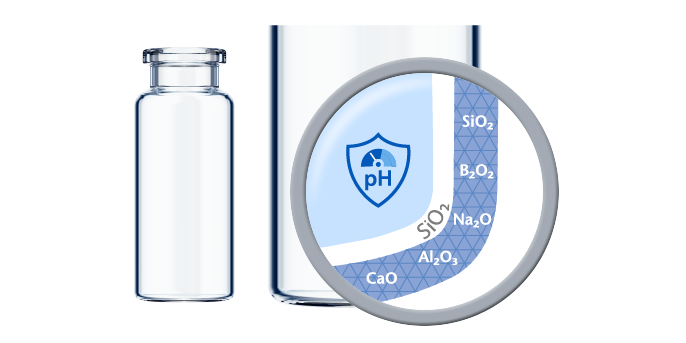
If predictive screening studies indicate a risk for delamination, EVERIC® pure is SCHOTT’s answer. Thanks to a leachable-improved glass tubing (FIOLAX® CHR: glass composition unchanged) and a patented hot-forming technology, SCHOTT Pharma manages to eliminate the production outliers with this changed heel zone. Without an additional coating or treatment, SCHOTT Pharma tackles the origin of the phenomenon by producing a vial with a homogenous inner surface.
The SCHOTT Quicktest (a dedicated, statistical in-production release test with defined limit values) controls that EVERIC® pure vials meet their specifications with regard to low leachables and delamination under full control. The continuously increasing demand and the trust of our customers are proof that EVERIC® pure vials are established as the gold standard against delamination.

Frequently asked questions about glass delamination
- Glass delamination in vials is a rare phenomenon and not easy to predict as there is no single factor that causes glass delamination. SCHOTT Pharma therefore offers predictive glass delamination screening studies based on USP <1660> to be performed prior to commercialization.
- The test procedure combines different analytical techniques as SEM cross-section analysis, stereomicroscopy, optical inspection of particles, ICP-MS, EDS and SIMS.